From starphenes to non-benzenoid linear conjugated polymers by substrate templating
Nanoscale Advances - 2021
Mohammed S. G. Mohammed and James Lawrence and Fátima García, Pedro Brandimarte, Alejandro Berdonces-Layunta and Dolores Pérez and Daniel Sánchez-Portal and Diego Peña and Dimas G. de Oteyza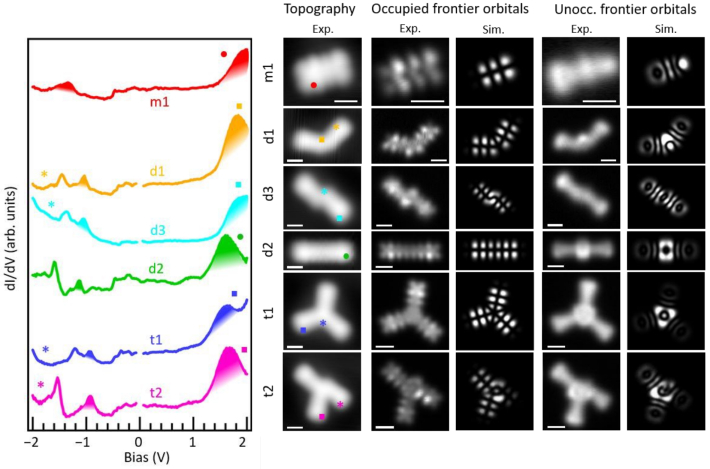
Abstract
Combining on-surface synthetic methods with the power of scanning tunneling microscopy to characterize novel materials at the single molecule level, we show how to steer the reactivity of one anthracene-based precursor towards different product nanostructures. Whereas using a Au(111) surface with three-fold symmetry results in the dominant formation of a starphene derivative, the two-fold symmetry of a reconstructed Au(110) surface allows the selective growth of non-benzenoid linear conjugated polymers. We further assess the electronic properties of each of the observed product structures via tunneling spectroscopy and DFT calculations, altogether advancing in the synthesis and characterization of molecular structures of notable scientific interest that have been only scarcely investigated to date, as applies both to starphenes and to non-benzenoid conjugated polymers.
Bibtex citation
@Article{Mohammed2021,
author = {Mohammed S. G. Mohammed and James Lawrence and F{\'{a}}tima Garc{\'{\i}}a and Pedro Brandimarte and Alejandro Berdonces-Layunta and Dolores P{\'{e}}rez and Daniel S{\'{a}}nchez-Portal and Diego Pe{\~{n}}a and Dimas G. de Oteyza},
title = {From starphenes to non-benzenoid linear conjugated polymers by substrate templating},
journal = {Nanoscale Advances},
year = {2021},
volume = {XXX},
number = {XXX},
pages = {1--21},
doi = {10.1039/d1na00126d},
publisher = {Royal Society of Chemistry ({RSC})},
}
Key words
- acene reactants
- on-surface synthesis
- conjugated polymers
- scanning tunneling microscopy
- density functional theory
- Tersoff-Hamann approximation